Pipeline
Cervello Therapeutics is focusing on enhanced Fasudil treatment which has resulted in decreased prevalence of Cerebral Cavernous Malformation (CCM) lesions1, a significant decrease in mature CCM lesion burden and improved survival in murine models of CCM2, with its therapeutic benefit having been indirectly attributed to inhibition of Rho Kinase (ROCK). Cervello Therapeutics goal is to develop novel derivatives of Fasudil with markedly improved ROCK potency and kinase selectivity, as well as enhanced properties for oral dosing, to better allow for interrogation of the role of ROCK inhibition in ameliorating CCM; our efforts have resulted in compounds showing > 100-fold stronger potency for ROCK inhibition than Fasudil and hydroxy-Fasudil.
Our efforts have resulted in a novel chemical series bearing little similarity to any other ROCK inhibitor in the literature and patent landscape. Lead compounds show >100-fold improvement in potency for ROCK inhibition over fasudil and hydroxy-fasudil, along with markedly improved selectively over the human kinome. We believe that the overall profile of these molecules positions them as uniquely suitable for a potential therapeutic to help existing patients heal CCM lesions, in both familial and sporadic cases of the disease.
We have selected CVT-100069 and CVT-100077 as development candidates that have both shown clear in vivo efficacy in terms of Cerebral Cavernous Malformation (CCM) lesion load in pre-clinical studies. Based on these findings, we are currently advancing CVT-100069 and CVT-100077 into IND-enabling studies.
Structural Basis for Design of Novel Derivatives of Fasudil
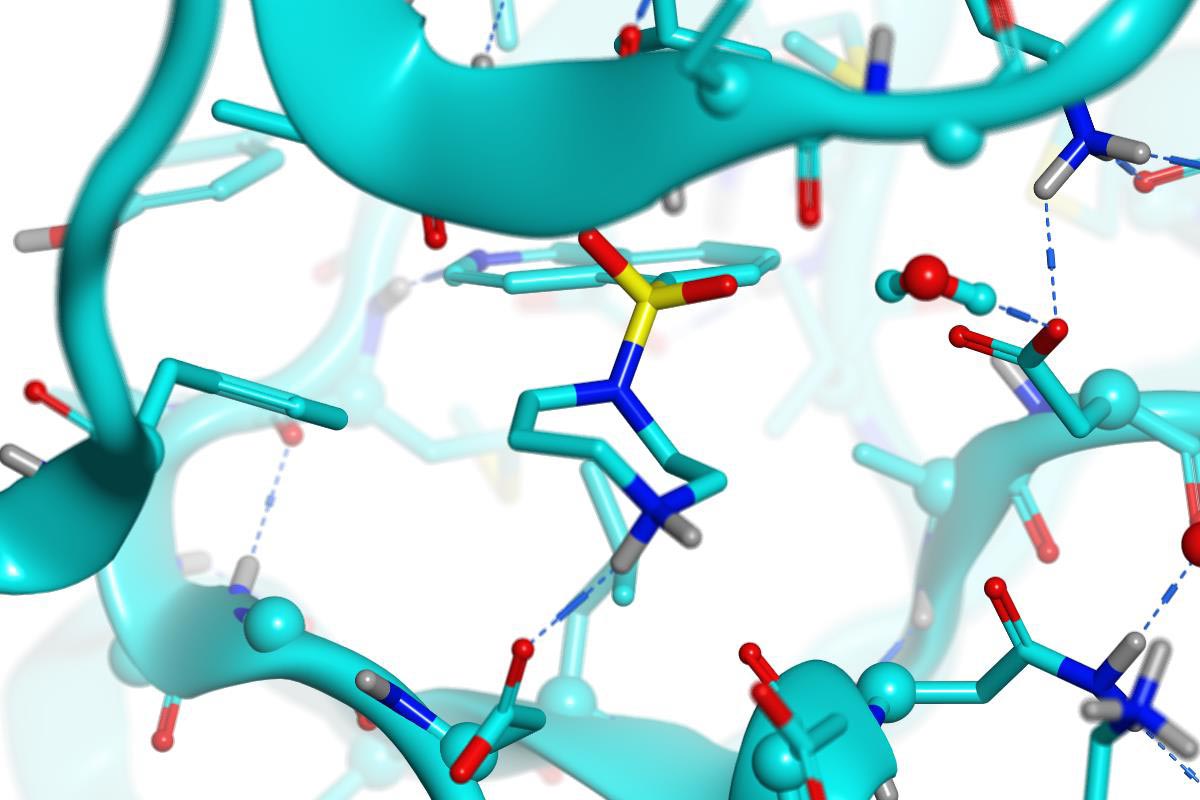
PDB 2ESM (3.20 Å) Fasudil in ROCKI
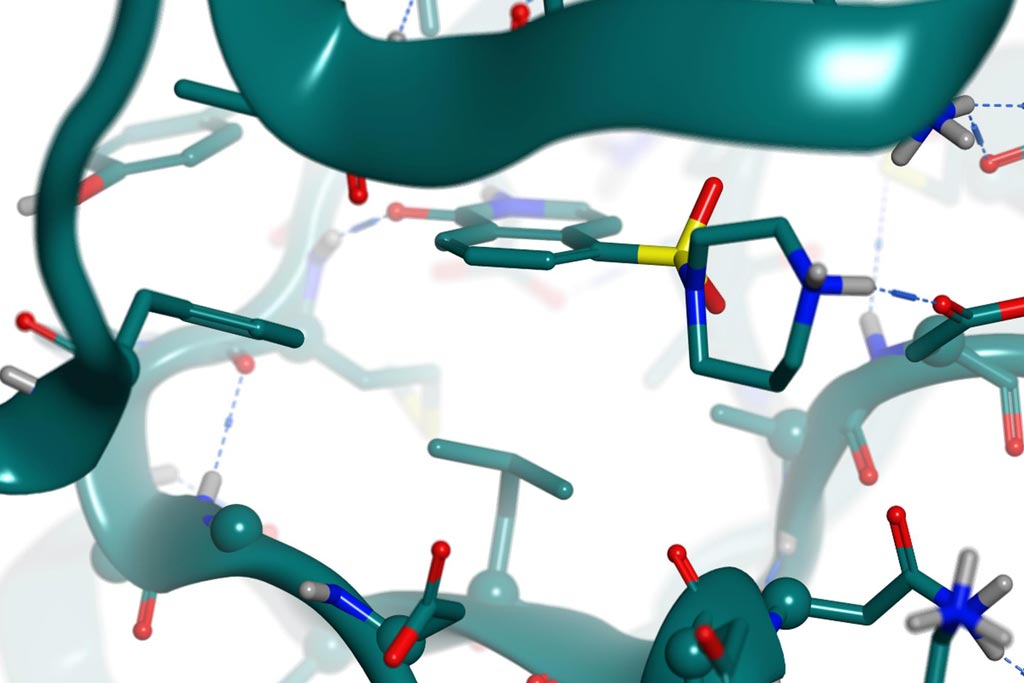
PDB 2ETK(2.96 Å) Hydroxy-Fasudil in ROCKI
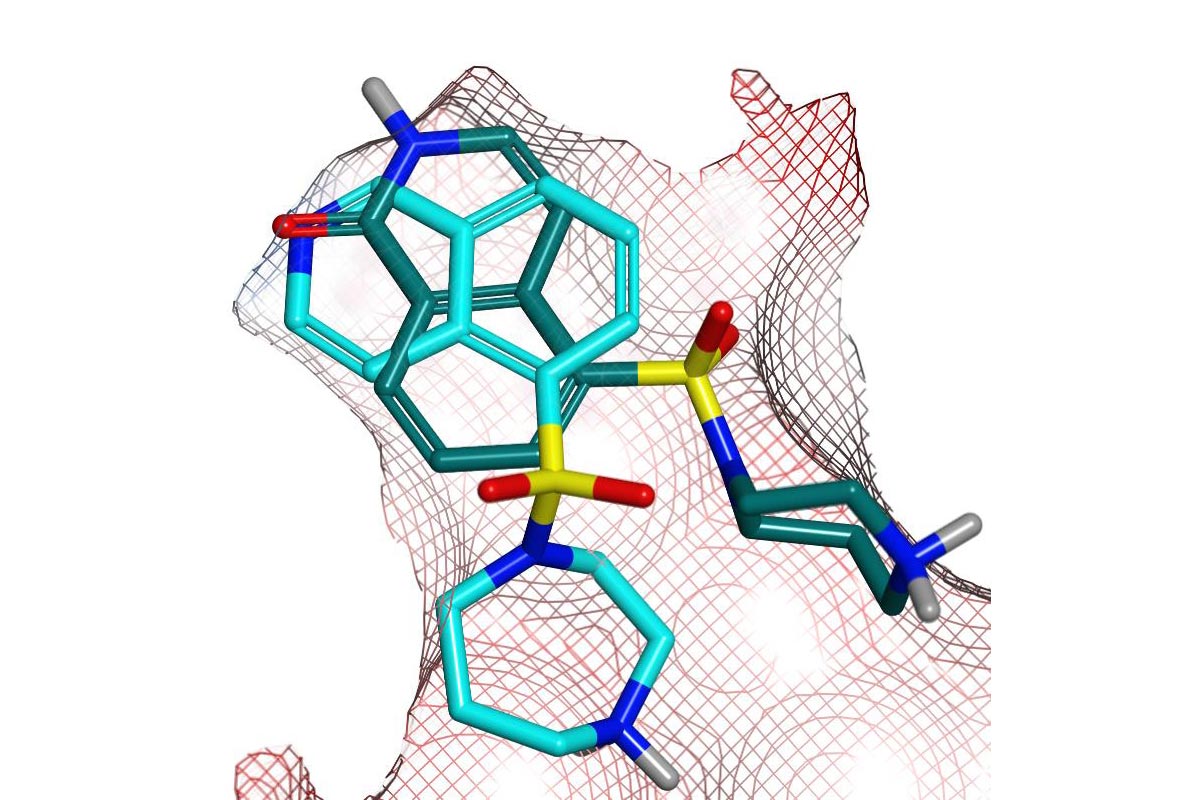
Fasudil & hydroxy-fasudil bind ROCKI with inverted binding modes.
Literature Reports of Fasudil Demonstrate Need for Improved Metabolic Stability
| CL int, in vitro | 36 ± 13 | μL/min/106 cells |
| mouse scaled CL int, in vitro | 359 ± 245 | ml/min/kg |
| human scaled CL int, in vitro | 122 ± 46 | ml/min/kg |
| In Vivo PK 3 | ||
| mouse CL t | 198 ± 15 | mL/min/kg |
| mouse t1/2 | 0.30 ± 0.1 | h |
| mouse CL int,in vivo | 1588 | mL/min/kg |
| human CLt | 73 | mL/min/kg |
| human t 1/2 | 0.26 | h |
| human CL int,in vivo | 371 | mL/min/kg |
Hydroxy-fasudil is the main metabolite and also bioactive.
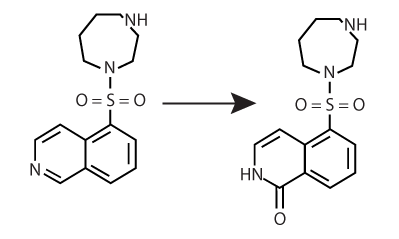
Structure-Based Design of Fasudil Analogs
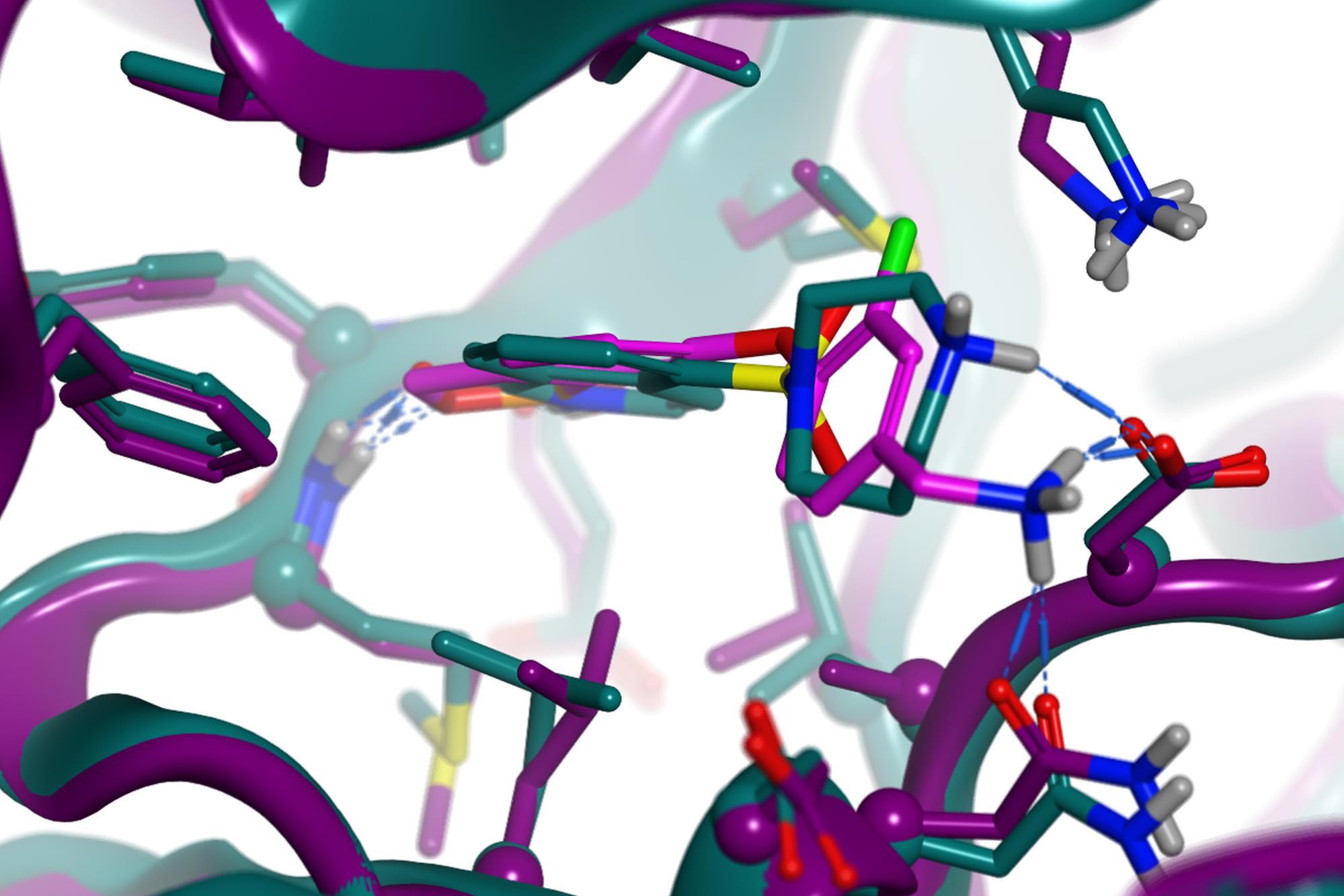
PDB 2ETK (2.96 Å) Hydroxy-fasudil in ROCKI
PDB 4L6Q (2.79 Å) benzoxaborole in ROCKII
PDB 4L6Q (2.79 Å) benzoxaborole in ROCK
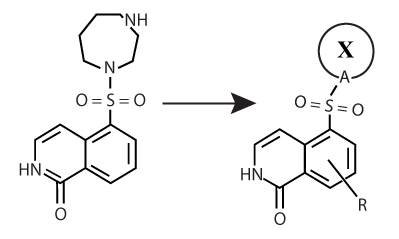
ROCKI & II crystal structures were used to design analogs of hydroxy-fasudil, with the primary objectives of improving potency & selectivity against the AGC kinase family.
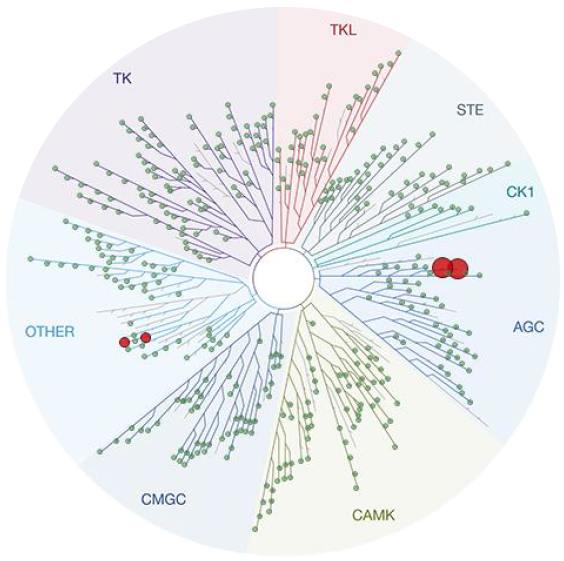
Cervello ROCKII, ROCKI, PKA, PKBα & PKG Data Illustrate a Need for Enhanced Kinome Selectivity
| Compound | ROCKII* | ROCKI* | PKA | PKA/ROCKII | PKBa (AKT1) | PKBa/ROCKII | PKG* | PKG/ROCKII |
|---|---|---|---|---|---|---|---|---|
| Fasudil | 351 | 476 | n/a | n/a | n/a | n/a | 3,529 | 10 |
| Hydroxy-fasudil | 252 | 476 | 7,030 | 28 | 2605 | 10 | 4,346 | 17 |
* IC50 values were determined with 14-point dose-response curves using a fluorescence coupling enzymatic assay using purified C-terminal truncated human ROCKI enzyme kinase domain (aa 1-477) fused to GST (Carna BioSciences, Catalog # 01-109) and purified C-terminal truncated human ROCKII enzyme kinase domain (aa 1-553) fused to GST (Carna BioSciences, Catalog # 01-110). Full length cGMP-dependent protein kinase 1 (PGK or PRKG1; aa 1-686) fused to GST (Carna BioSciences, Catalog # 01-142) was used as a representative control for offtarget kinase activity within the AGC-family.
Hydroxy-fasudil is the main metabolite and also bioactive.

CVT-100069 at 400 nM
| Compound | ROCKII | ROCKI |
|---|---|---|
| Hydroxy-fasudil | 4100 | 2400 |
| CVT-100069 | 18 | 29 |
| CVT-100077 | 16 | 16 |
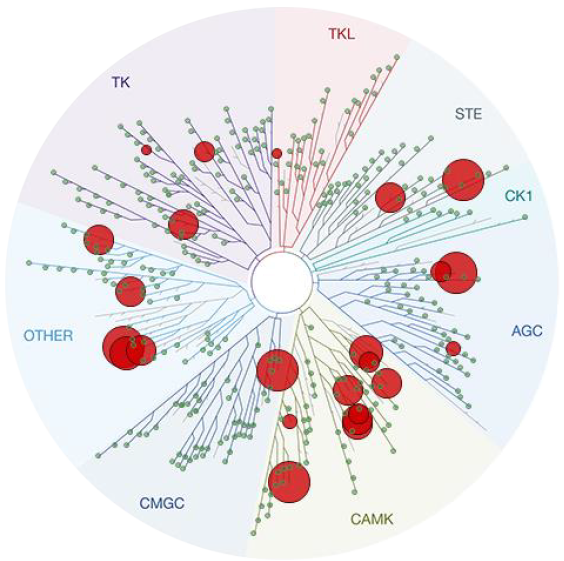
403 non-mutant kinases in panel
(including ROCKI/II)
CVT-100077 at 400 nM
-
S35 = 0.5% (2/401)
S10 = 0.0% (0/401)
| 0% | |
| 0.1% | |
| 0.1 - 1% | |
| 1 - 5% | |
| 5 - 10% | |
| 10 - 35% | |
| > 35% |
-
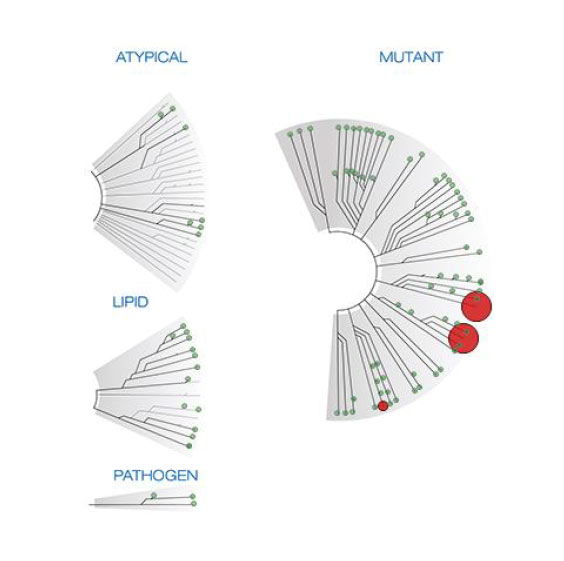
-
S35 = 5.5% (22/401)
S10 = 5.0% (20/403)
Literature Reported Activities of Fasudil4 & hydroxy-Fasudil5 Also Illustrate the Need for Enhanced Kinome Selectivity
| Compound | ROCKIII | PKA | PKA/ROCKII | PKC | PKC/ROCKII | PKG | PKG/ROCKII |
|---|---|---|---|---|---|---|---|
| Fasudil | 330 | 1,600 | 5 | 3,300 | 10 | 1,600 | 5 |
| Hydroxy-fasudil | 720 | 37,000 | 51 | N/A | N/A |
Neither of these benchmark compounds are highly selective against the limited # of AGC kinases against which they have been profiled. Biological activity attributed to these benchmarks in cellular, tissue & in vivo assays may result in part from off-target kinase activity.
Cervello Passive Permeability & Efflux Data Demonstrates Need for Improved ADME Properties over Fasudil & hydroxy-Fasudil
| MDCK Passive Permeability | MDCK Efflux | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | WT Papp ,A-B (10 -6 cm/s) |
OE MDR1 Papp,A-B (10 -6 cm/s) |
OE BCRP Papp,A-B (10 -6 cm/s) |
WT Efflux Ratio | OE MDR1 Efflux Ratio | OE MDR1 EER | OE BCRP Efflux Ratio | OE BCRP EER |
| Metoprolol | 35.9 | 29.3 | 34.6 | 0.8 | 0.8 | 1.0 | 0.8 | 1.1 |
| Atenolol | <0.2 | 1.0 | 0.008 | >0.4 | 0.7 | n/a | 1.3 | n/a |
| Terfluonomide | 2.7 | n/a | 17.6 | n/a | ||||
| Hydroxy-Fasudil | 0.8 | 2.1 | 0.6 | 1.5 | 3.4 | 2.3 | 8.5 | 5.9 |
- 1. Stroke,43:571-574 (2011)
- 2. Stroke,48:187-194 (2016)
- 3. Drug Metabolism & Disposition, 40:322-328 (2012); Asian Journal of Pharmacodynamics and Pharmacokinetics, 9:221-226 (2009)
- 4. Pharmacol Ther, 82:123-131 (1999)
- 5. Stroke, 36:2251-2257 (2005)